Information for Patients
The decision to participate in a clinical trial can be an emotional one, and often brings up more questions than answers, at least initially.
As a patient, it is important for you to make an informed decision about the next steps in your treatment and communicate your decision with those closest to you.
We are committed to being both intentional and representative when it comes to the inclusion of diverse populations of patients in the disease areas we are studying. To us, diversity in clinical trial participation means that the clinical trial patient population should, to the extent possible, reflect the diversity of the intended patient population once the medicine is approved.
We are also determined to understand the prevalence of the disease in diverse populations, so we can deliver solutions that enable equitable trial access and enhanced trial experiences to the patients we serve. This may include but is not limited to people of different races, ethnic groups, sex/genders and ages, disabilities or geographies.
Each and every participant in a clinical trial broadens the pool of information available to researchers in pursuit of new and novel treatments. Your participation matters, and will have long-lasting impact.
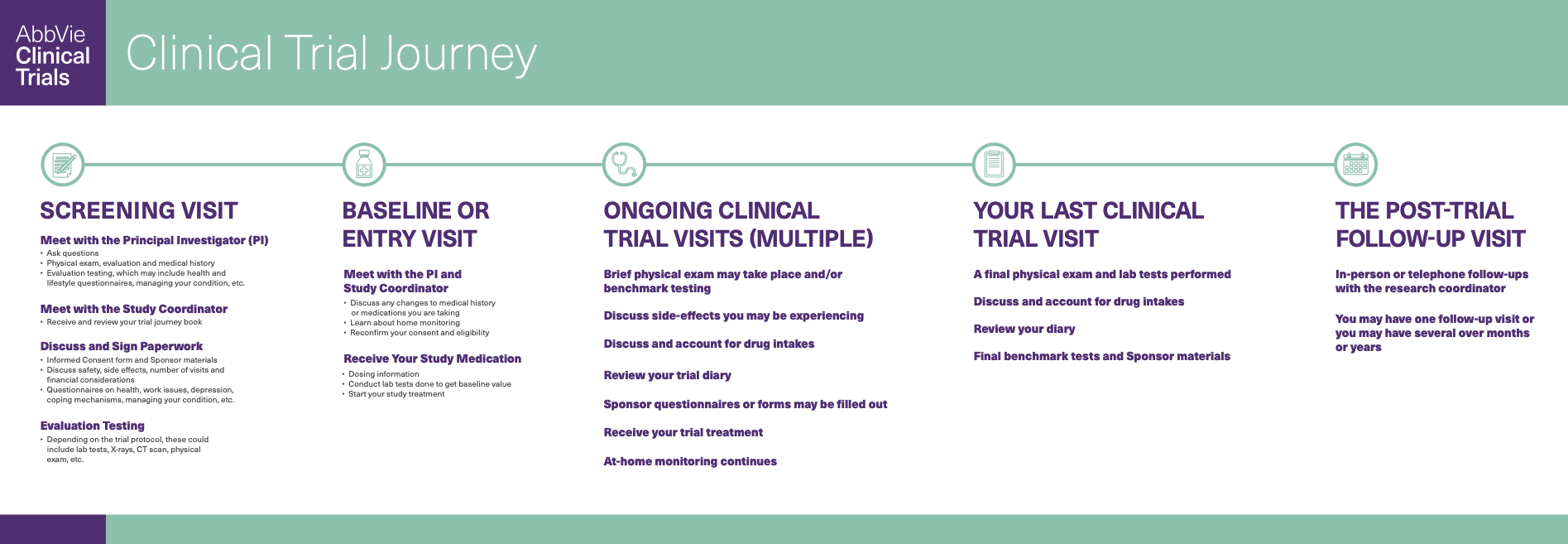
The first step is understanding how to find clinical trials for your disease or condition and whether a clinical trial is right for you. Please see the list of resources related to clinical trial patient recruitment to give you as much information as possible to make the right choice for you.
EXPLORE FAQS AND GUIDANCE
Why participate in a clinical trial?
ARE YOU INTERESTED IN RESULTS OF COMPLETED ABBVIE CLINICAL TRIALS?
Reading about past clinical trials — how they were conducted and their results — can give you insights into the clinical trial journey. That's why AbbVie is sharing summaries of completed trials and making them available in a dedicated online library.
These completed trial results — you may also see them being called plain language summaries or lay person summaries — are brief descriptions of the clinical trial results in everyday language that is understandable to people who do not have a medical or scientific background. You can expect descriptions of what was studied and general results, and we also believe this information will show the valuable role participants play in the process.
To learn more about the results, click on the link to completed trials below.