As you have learned more about the potential risks and benefits of participating in a clinical trial and considered whether or not it is right for you, you may have asked yourself, are clinical trials ethical?
There are seven main guidelines for ensuring that clinical trials are performed in an ethical way.
Organizers of a clinical trial are required to answer all of the following questions affirmatively:1
Social and clinical value: Does the trial answer a specific question? Does answering this question have a significant value for society or for future patients?
Scientific validity: Is the trial’s question answerable (i.e., can it be tested) and are the research methods valid and feasible? Is the trial designed with clear objectives, principles, methods, and practices?
Fair subject selection: Are the trial participants appropriately chosen to address the scientific questions being asked?
Favorable risk-benefit ratio: Are the benefits of participating worth the risks? Have steps been taken, in the trial, to minimize risks and maximize benefits?
Independent review: Has an unbiased independent panel, free of any conflicts of interest, reviewed the plan for the trial?
The final two guidelines involve more participant-focused issues and include patient’s rights, clinical trial confidentiality, and informed consent in clinical trials.1
To learn more about the potential risks and benefits of participating in a clinical trial, read "Are clinical trials safe?"
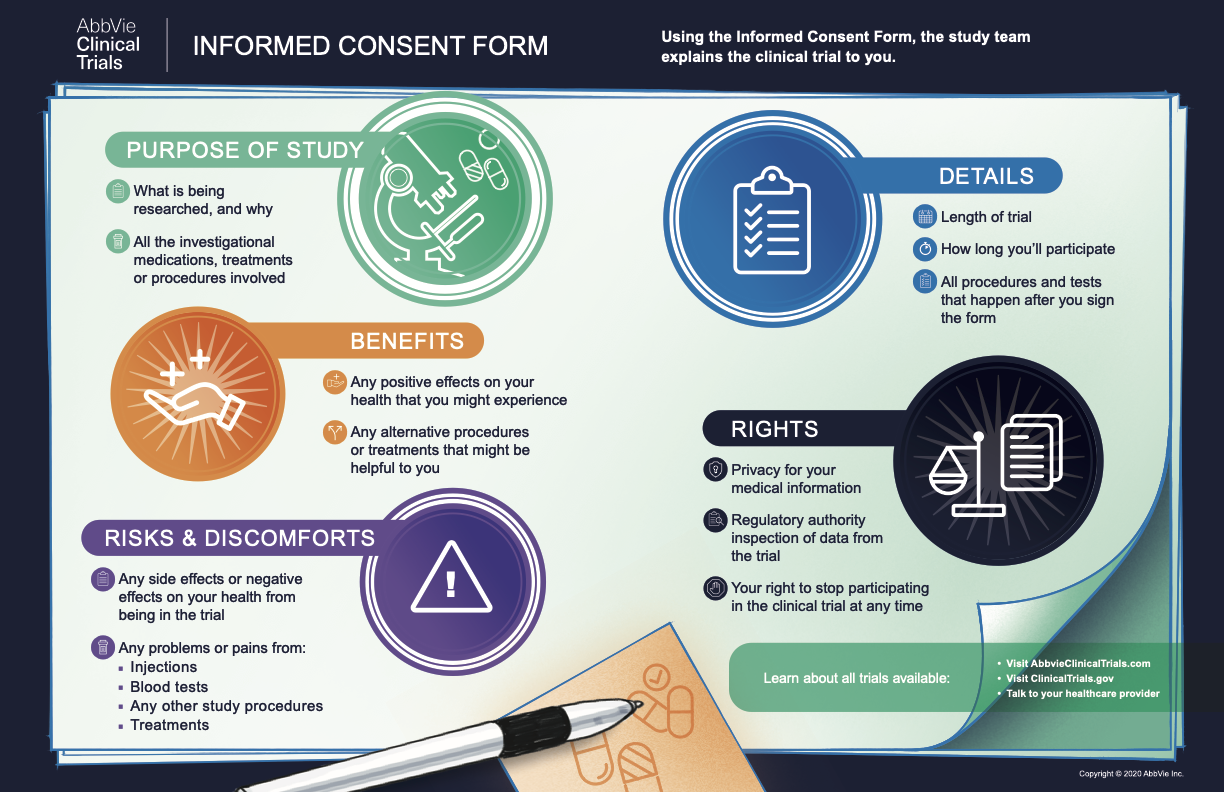
ARE YOUR RIGHTS PROTECTED? WHAT IS INFORMED CONSENT IN CLINICAL TRIALS?
As a potential clinical trial participant, you have the right to fully understand the procedures and purpose of a specific trial before you agree to participate. A process called informed consent is in place to ensure that you are aware of all necessary information.2 Maintaining informed consent in clinical trials is an ongoing process, and the doctors or nurses running the trial will inform you about the trial’s key facts before you enroll, and they are responsible for informing you of any relevant information throughout your participation.
Once all of your questions about the trial have been answered, you will be asked to sign a form, called informed consent in clinical trials. This form confirms that you agree to participate in the trial. Feel free to ask any additional questions, clarify any language that you don’t understand, and/or take the document home to discuss your decision with your loved ones before you sign.
Your Rights Guaranteed by Informed Consent
You have the right to ask at any time about any aspect of the trial, including procedures, adverse reactions, or anything else.3
You are entitled to keep all of your medical information, whether collected in the past or during the clinical trial, confidential and private.
The only people who will have access to your personal medical data are the doctors and personnel who are conducting the trial, the ethics committee reviewing the trial, trial sponsor representatives who ensure the trial is conducted properly, and in some cases, local and foreign regulatory authorities. However, this data is never attached to your name or any other identifying information that would reveal your identity and its connection with specific clinical data.
Your participation in a clinical trial is completely voluntary and you can withdraw for whatever reason, at any time.3
If you decide to withdraw, you may be able to discuss alternative treatment options with the clinical trial doctor. Through collaboration between the clinical trial doctor and your personal healthcare professional, your treatment team can avoid repeating certain tests and ensure that you get the best possible care, whether in the trial or not.
You also have the right to request access to your clinical trial data and records.3
However, to ensure clinical trial confidentiality, only a few select people are allowed to see the data before the end of the trial. Sharing these data with you, or anyone else, before the trial ends can compromise the validity of the study and jeopardize your confidentiality and privacy. If you request to see your clinical trial data, researchers may have to wait until trial results are made public before sharing the information with you. As mentioned above, your individual data will be kept confidential and never be attached to any identifying information, such as your name.
THE RIGHT CHOICE
Clinical trials are necessary to determine the risks and benefits of a new potential treatment in a group of human volunteers. Giving your informed consent to participate and knowing your rights while participating are important parts of ensuring that the clinical trial process is ethical. There are many other safeguards in place to ensure clinical trial confidentiality and protect clinical trial data. You and your treatment team may decide that a clinical trial is not the right decision for you. Be sure to ask questions about your participation and to understand your rights in the clinical trial process, including the right not to participate.