Clinical Trial Journey
Whether you are gathering information about a potential research study or have just volunteered for a clinical trial, your clinical trial journey is as unique as the reasons behind your choice to participate.
HOW MANY CLINICAL STUDY OPPORTUNITIES ARE THERE?
A big consideration for both researchers and participants is eligibility to participate. Interest and willingness are not the only requirements for eligibility. The ideal clinical trial volunteer will be representative of the eventual patient who will take the drug or therapy being tested. Finding this match between trial and volunteer is important so that reviewers, researchers, clinicians, and governing agencies can have confidence that the trial results will be applicable to the intended patients for the intended condition.1
Clinical trial volunteers are essential to further drug development and research. Our commitment to understanding the prevalence of the disease in diverse populations, helps us to deliver customer-centric solutions that will offer equitable trial access and enhanced trial experiences for any participant, in any population group.
This clinical trial journey will take you through some of the typical benchmarks of a trial and provide insights to help you make a decision about whether or not volunteering for a clinical trial is right for you.
CLINICAL STUDY VOLUNTEER EXPERIENCE AND TIMELINE
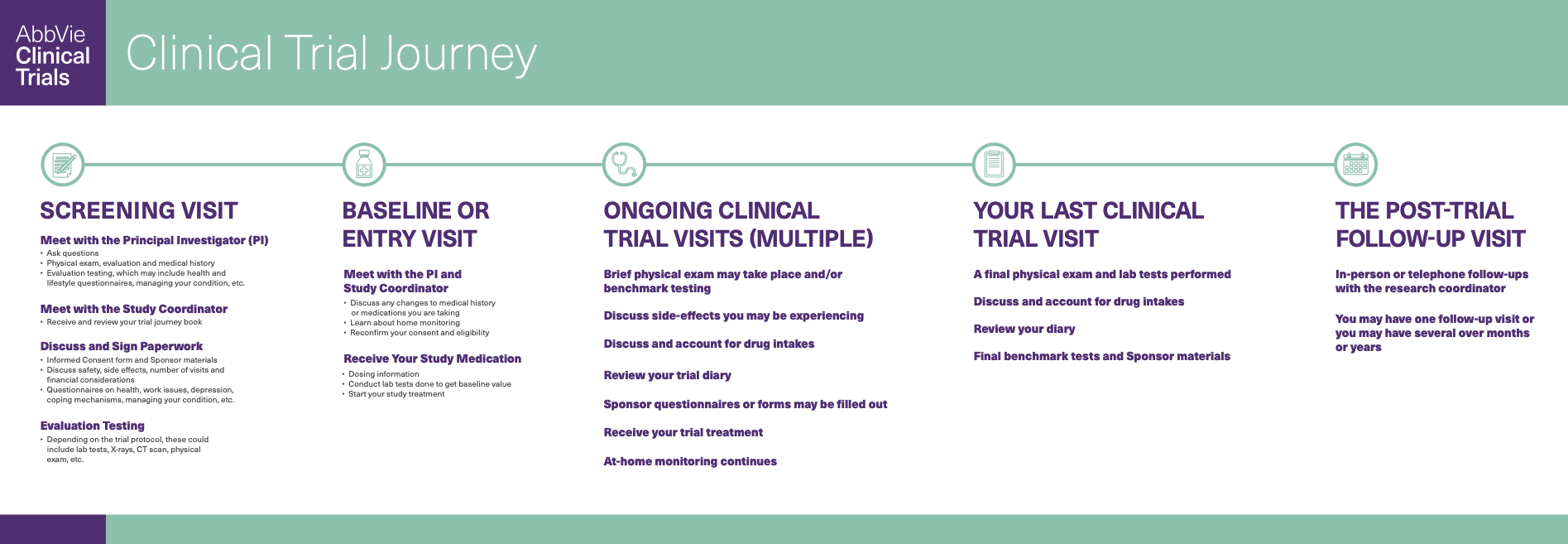
The clinical trial journey is different for everyone, depending on your current state of health, your age, the type of trial, the duration of the study, and many other factors. Yet in many ways, the standard clinical trial experience is largely the same for most clinical study volunteers. A typical clinical trial timeline—including all visits and what a participant's trial journey looks like from start to finish—involves decisions and engagement opportunities for patients, families and health care providers at every step of the way.
The various milestones characteristic of a clinical study, which combined, make up the clinical trial participant journey, are explained below.
1. The Screening Visit
When a clinical study volunteer first attends the screening visit for phases 1-3, the clinical trial is explained in detail with opportunities to ask questions.
The study staff will review the informed consent form, which describes the purpose of the clinical research, eligibility and your rights as a participant. Once you are confident in your decision to participate, you will be asked for your signature and permission to proceed with the screening evaluations. Even if you sign the form at this initial visit, the researchers conducting the study will provide you with the opportunity to take the informed consent form home to discuss it with your family or caregivers before returning it. If you choose to sign and submit your informed consent form on the same day as your screening visit, your clinical trial could begin that day.
In addition to an interview to establish eligibility, the screening visit is designed to provide enough information to enable the researchers to decide with confidence whether you are a good candidate for the given study. No tests can be performed before the informed consent form is signed.2
2. The Entry or Baseline Visit
If the screening tests show that you qualify for the clinical trial, the study coordinator will schedule an entry, or baseline visit. In general, the baseline visit takes place after the screening visit since the focus of the screening visit is to establish whether or not a person meets the criteria to participate.
You could be asked to submit to a variety of tests to obtain baseline values before starting your study treatment. These tests may include:
Height, weight and BMI (body mass index) measurements
Temperature and blood pressure
A urine sample
Vital signs and ECG
Physical exam
Blood samples2
When you attend the baseline visit, you may receive your study medication. The dosing schedule will be reviewed with you along with any other study responsibilities.
3. Clinical Trial Visits
The next set of clinical trial visits make up the study treatment period. At each visit, participants will go through a set of events dictated by the clinical trial protocol. A clinical trial protocol is a document that outlines the standardized objectives and organization of a clinical trial to ensure that the trial can be conducted the same way at each site, and that the trial is safe and ethical.3
The number of clinical visits will depend on the specifics and length of the trial. Generally, each visit may include a brief physical exam (with the exception of phase 4 observational studies), a review of your study medications and your symptoms, and lab tests.
Rarely, a clinical trial may end abruptly. There are various reasons for this, but your clinical trial investigator will explain the reasons for stopping a study, what it means for you, and what your options are. In addition, the trial investigator will probably request you follow-up with your health care provider to find out about additional options available to you.
4. The Last Clinical Trial Visit
The last clinical trial visit means that the study treatment period is over. This visit doesn’t always mean the journey’s end; the trial may require follow-up visits. While you will no longer be requested to perform at-home monitoring, you may be asked to check in with the investigator in the weeks and months ahead, and to return to your health care provider for continued treatment and/or checkups.
Participants should ask when the results of their clinical trial will be available, and how they will be informed. While your part may be over, the clinical trial itself can last longer.
At this point, volunteers can ask about additional follow-ups and if there will be any future studies for which they may qualify.
Every completed clinical trial will most likely be the focus of a published research paper. This paper is directed at fellow researchers to inform the design of future studies for a particular disease state or condition. Many of these papers are “open access,” which means they are openly accessible and free to the public to read and download. You can search for results from your clinical trial and the results of other clinical trials for a particular disease, indication or marker on PubMed Central® (PMC), a free full-text archive of biomedical and life sciences journal literature housed in the United States National Institutes of Health's National Library of Medicine (NIH/NLM). You can also easily access information about publicly or privately funded clinical studies on ClinicalTrials.gov. The information on this site is provided and updated by the sponsor or principal investigator of the clinical trial. If you are unsure about how to interpret the results of your study or another study, you can contact your clinical trial investigator or your health care provider for help.
5. The Follow-Up Visit
The clinical study volunteer follow-up visit may take place weeks, months, or rarely, years after the last clinical trial visit.
It is important to note that the follow-up visits for a clinical trial are just as important as the clinical trial visits that took place during the study. In some cases, the loss of participants during the follow-up stage can affect the validity of the study results, especially if the loss is >20%.4

SCREENING VISIT
Meet with the Principal Investigator (PI)
Meet with the Study Coordinator
Discuss and Sign Paperwork

BASELINE OR ENTRY VISIT
Meet with the PI and Study Coordinator
Receive Your Study Medication

ONGOING CLINICAL TRIAL VISITS (MULTIPLE)
Brief physical exam may take your place and/or bench mark testing
Discuss side-effects you may be experiencing
Discuss and account for drug intakes
Review your trial diary
Sponsor questionnaires or forms may be filled out
Receive your trial treatment
At-home monitoring continues

YOUR LAST CLINICAL TRIAL VISIT
A final physical exam and lab tests performed
Discuss and account for drug intakes
Review your diary
Final benchmark tests and Sponsor materials

THE POST-TRIAL FOLLOW-UP VISIT
In-person or telephone follow-ups with the research coordinator
You may have one follow-up visit or you may have several over months or years
References:
Califf, M.D., Robert M. 2016: The Year of Diversity in Clinical Trials. FDA Voice. (Jan 27, 2016) Retrieved from https://blogs.fda.gov/fdavoice/index.php/2016/01/2016-the-year-of-diversity-in-clinical-trials/
Brenda Wright, Chapter 14 - Screening, Treatment, and Safety Follow-up Visit, In A Comprehensive and Practical Guide to Clinical Trials, Academic Press, 2017, Pages 133-154, ISBN 9780128047293, https://doi.org/10.1016/B978-0-12-804729-3.00014-6.
Clinical Trials Protocol Template. National Institutes of Health. (April 24, 2018). Retrieved from https://grants.nih.gov/policy/clinical-trials/protocol-template.htm
Joseph R. Dettori. Evid Based Spine Care J. 2011 Feb; 2(1): 7–10. doi: 10.1055/s-0030-1267080 PMCID: PMC3427970 PMID: 22956930
SCREENING VISIT: A VIRTUAL TOUR OF A CLINICAL TRIAL RESEARCH SITE
Experience a virtual tour that takes you inside an example of a clinical research trial site and shows you the kind of clinic environment, people and activities you may encounter during a screening visit.